The visit of the Ukrainian delegation headed by Deputy Prime Minister for the reintegration of the temporarily occupied territories of Ukraine Oleksiy Reznikov to Israel resulted in the solution of a number of important issues for the country, in particular, to overcome the COVID-19 pandemic. The corresponding statement was made by the Ambassador of Ukraine to Israel Yevhen Korniychuk on his Facebook page.
“I want to thank all the delegates for their active position in the work and significant contribution to the issue of reaching agreements in cooperation with Israel. Together, we solved a number of important issues for our country, in particular, interaction in the field of trade, investment, medicine and in overcoming the COVID-19 pandemic,” the diplomat said.
He said that an important aspect of the negotiations was the completion by Israel of the second phase of trials of its COVID-19 vaccine in mid-summer.
“The Ukrainian side proposed to conduct the third stage of testing in Ukraine with the subsequent possibility of producing a vaccine, including at our facilities. Israel is still studying this proposal and, I hope, it will be received positively. It is already clear that the pandemic of coronavirus infection will drag on for more than one year in the world. And the production of the vaccine, its availability for our citizens is a very sensitive and important issue for us,” Korniychuk said.
Pharmaceutical company Lekhim (Kyiv) as an official representative of the Chinese vaccine manufacturer Sinovac Biotech plans to transfer the first batch of the Chinese CoronaVac vaccine against coronavirus (COVID-19) disease to state-owned enterprise Medical Procurement of Ukraine after the completion of laboratory control on Saturday.
“The laboratory control ends tomorrow. We have no result yet, it will be tomorrow, and tomorrow we will deliver the vaccine to the Medical Procurement of Ukraine. This issue has already been settled, they confirmed the delivery address,” the company told Interfax-Ukraine on Friday.
Lekhim said that the Medical Procurement of Ukraine will be engaged in further delivery of the vaccine.
In addition, the company confirmed negotiations to extend the contract with Sinovac by another 5 million doses.
“The issue of increasing the volume of contact has been discussed, but there is still no concrete answer from the Chinese side. We expect information from them next week, but the already purchased 1.9 million doses should be delivered, although the terms are constantly shifting,” the company said.
As reported, on April 8, the Medical Procurement of Ukraine confirmed to Lekhim its readiness to accept the supply of the COVID-19 vaccine produced by Sinovac Biotech Ltd. under a procurement agreement for the national budget’s funds.
State-owned enterprise Medical Procurement of Ukraine has confirmed to the Lekhim company its readiness to accept the supply of vaccine against coronavirus (COVID-19) manufactured by Sinovac Biotech Ltd. under a procurement agreement for the national budget’s funds.
The Health Ministry of Ukraine told Interfax-Ukraine, Lekhim provided the state-owned enterprise with all the necessary documents that are required when informing about the shipment of products.
Among these documents, in particular, an invoice, a certificate of quality (analysis) of a series of products, a marketing authorization, a certificate of origin from the manufacturer, information on WHO retraining and/or a marketing authorization from a competent authority of one of the designated countries, a packing list and a document confirming adherence to the cold chain and others.
Medical Procurement of Ukraine says that the documents were handed over by the supplier on Tuesday.
“Having processed the documents sent by the supplier company yesterday, the Ministry of Health confirmed to the counterparty its readiness to accept the batch of products specified in the supplier’s documents in the amount of 200,000 bottles/syringes,” the Health Ministry said.
At the same time, the state-owned enterprise says that “the implementation of this delivery does not release the supplier from the obligation to pay a penalty and fine.”
According to the Health Ministry, a penalty for each day of delay will be charged starting March 6, 2021.

The United States Agency for International Development USAID will provide Ukraine with special refrigerators necessary for transporting and storing the Pfizer vaccine, the press service of the U.S. Embassy in Ukraine said.
“To support Ukraine’s national response, USAID is helping prepare for Pfizer and other approved vaccines by providing direct support for ultra-cold chain storage/transportation and supporting public communications efforts to provide accurate information about vaccines,” the message reads.
As reported, Ukraine signed an agreement with the American pharmaceutical corporation Pfizer for the supply of 10 million doses of vaccine against COVID-19, which was agreed in February by Ukrainian President Volodymyr Zelensky and chief executive officer of Pfizer Albert Bourla.
The second batch of the coronavirus (COVID-19) vaccine from the Chinese manufacturer Sinovac Biotech is expected in the coming weeks, Ukrainian President Volodymyr Zelensky said.
He said that the signed contract provides for the receipt of almost 2 million doses, according to the presidential press service.
“In addition to China, the Sinovac vaccine is already used by 18 countries of the world. In particular, Turkey, where President Erdogan and Patriarch Bartholomew were vaccinated with it,” Zelensky said.
As reported, on Thursday evening, March 25, the first batch of the Chinese COVID-19 vaccine CoronaVac, produced by the Chinese company Sinovac Biotech, in amount of 215,000 doses, arrived at Boryspil International Airport.
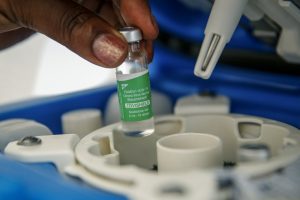
The independent laboratory Invitro will conduct research on the effectiveness of the vaccine CoviShield/AstraZeneca, a press release of the company says.
Head of the laboratory’s clinical diagnostic complex Pavlo Naboka said that the study will consist in checking the amount of antibodies that were produced after vaccination.
“According to the official instructions, the principle of action of the CoviShield vaccine is based on the development of specific immunity to the S-protein (spike) of the SARS-CoV-2 coronavirus (the causative agent of COVID-19). The presence of a sufficient amount of antibodies to this protein is a factor that protects a person from the development of the disease even in case of infection. It is possible to check how many antibodies have developed in the body after vaccination by using a new laboratory test by the world famous manufacturer Abbott Diagnostics to quantify the level of neutralizing (protective) antibo
During the entire COVID-19 vaccination process, blood samples will be taken from laboratory staff to monitor immune levels, he said. That is, the laboratory will assess how many antibodies to SARS-CoV-2 will appear in a person after the first and second vaccinations, at what rate of antibodies will be produced, whether the actual concentration of antibodies will correspond to the data reported by the vaccine manufacturer.
According to the instructions, a sufficient level of antibodies should be formed within 90 days after the first administration of the vaccine. That is, the laboratory Invitro will be able to announce the results of the study in the summer of 2021.
AstraZeneca, COVISHIELD, EFFECTIVENESS, INDEPENDENT LABORATORY, VACCINE