
DTEK Renewables has commissioned the 240 MW Pokrovska solar plant (Nikopol district, Dnipropetrovsk region).
The press service of the company said Pokrovska solar plant is a project implemented by Ukrainian companies and specialists. Some 16 enterprises took part in the construction, which received orders worth EUR48 million.
Pokrovska solar plant consists of 840,000 solar panels manufactured by Risen (China). It is expected that each year the plant will produce 400 million kWh of “green” electricity, which is enough to provide 200,000 households.
“Pokrovska solar plant is the company’s third project in solar energy, investment in which amounted to EUR193 million,” DTEK CEO Maksym Tymchenko said.
He said that the payback period in current tariffs is estimated at six to six and a half years.
In addition, he said that DTEK plans to implement the project for building the Energy Storage system either at Pokrovska or Nikopol solar station.
National bank of Ukraine’s official rates as of 01/11/19
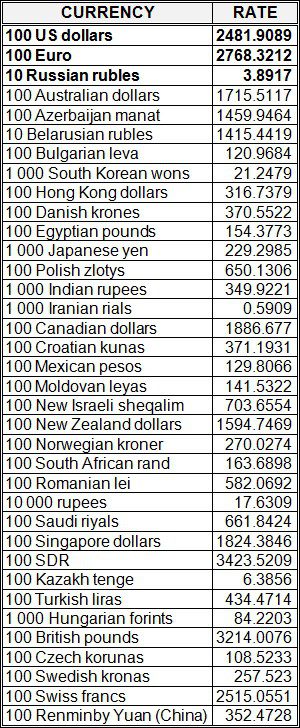
Source: National Bank of Ukraine

The State Automobile Roads Agency (Ukravtodor) and China’s Poly Changda Engineering Co. Ltd have signed a memorandum of cooperation as part of the implementation of the first phase of construction of Big orbital roads around Kyiv connecting M-05 Kyiv-Odesa and M-06 Kyiv-Chop highways.
According to a posting on the website of the Infrastructure Ministry, during signing the document, Infrastructure Minister Vladyslav Krykliy said that the memorandum is an important stage in cooperation for the start of construction of the orbital road.
“Our goal is to ensure 40% growth of the country’s economy in five years, and for this we need to ensure large-scale construction throughout the country. Any infrastructure project gives a return with the greatest multiplier. Each dollar invested today in a year will give plus $2 for the economy. We are very pleased that Poly Changda Engineering shares our big investment plans, and especially in such important projects as the Great Orbital Road around Kyiv. In Ukraine, there was no experience of concession roads, so it is very important to start these projects with large reliable strategic partners,” the press service said, citing Krykliy.
At the same time, Krykliy expressed his expectations that both sides will be able to quickly complete all the required preparatory technical and economic calculations and begin construction next year.

State-owned enterprise (SOE) Skhidny (Eastern) Mining and Processing Plant (Dnipropetrovsk region) in January-September 2019 cut production of uranium concentrate by 14.9% year-over-year, to 684.8 tonnes, according to information on the unified government portal of open data.
According to the enterprise, 72.6 tonnes of uranium concentrate were produced in January, 93.7 tonnes in February, 84.3 tonnes in March, 72.9 tonnes in April, 52.2 tonnes in May, and 80 tonnes in June, in July – 72.2 tonnes, in August – 84 tonnes, and in September – 72.9 tonnes.
As reported, Skhidny Mining in 2018 increased the production of uranium concentrate 41.1% compared to 2017, to 1,179.5 tonnes.
Skhidny Mining is the only uranium ore mining and processing enterprise in Ukraine and the largest in Europe. The annual needs of Ukrainian nuclear power plants for uranium concentrate are about 2,400 tonnes, while its production by Skhidny Mining is about 1,000 tonnes.

The net profit of state-owned Oschadbank (Kyiv) in January-September 2019 totaled UAH 189.017 million, which is a 2.6-fold rise from a year ago (UAH 72.938 million), according to quarterly financial statements of the bank.
According to the statements posted on the bank’s website, net profit in Q3 2019 amounted to UAH 64.106 million, which is 52.9% more than in Q3 2018.
Net interest income for in January-September 2019 increased 1.2% compared with the corresponding period of 2018, to UAH 4.185 billion.
In January-September 2019, the bank’s assets decreased 4.4%, to UAH 208.109 billion, including a decline of 3.5% in loans issued to customers, to UAH 65.146 billion.
Assets for this period increased 0.4%, to UAH 18.619 billion.
The charter capital for the period remained at the level of UAH 49.725 billion.
Oschadbank was founded in 1991. Its sole owner is the state.
According to the National Bank of Ukraine, as of July 1, 2019, Oschadbank ranked second (UAH 288.339 billion) among 76 banks operating in the country in terms of total assets.