The Ministry of Health of Ukraine has supplied innovative drugs for COVID-19 to the regions, in particular 20,000 bottles of Bamlanivimab and 40,000 bottles of Etesevimab, the Ministry said with reference to Deputy Health Minister for European Integration Oleksandr Komarida.
“In June, the working group made a decision on the readiness to include in the treatment protocol [for patients with COVID-19] the drugs Bamlanivimab and Etesevimab, if available in Ukraine. Today, the drugs are already in the regions, and accordingly the updated protocol will regulate their use,” said Komarida.
The drugs were delivered to Ukraine on September 4 with the assistance of the Ukrainian Embassy in the United States as a humanitarian aid provided by the American charitable organization Direct Relief.
According to Health Minister Viktor Liashko, the latest agreements on receiving this assistance were agreed upon during the visit of Ukrainian President Volodymyr Zelensky to the United States.
The ministry notes that the mechanism of action of the drugs is to block the attachment of the virus to the cell membrane, which makes it impossible for SARS-CoV-2 to enter human cells, neutralizes the effect of the virus and helps prevent the development and treatment of COVID-19.
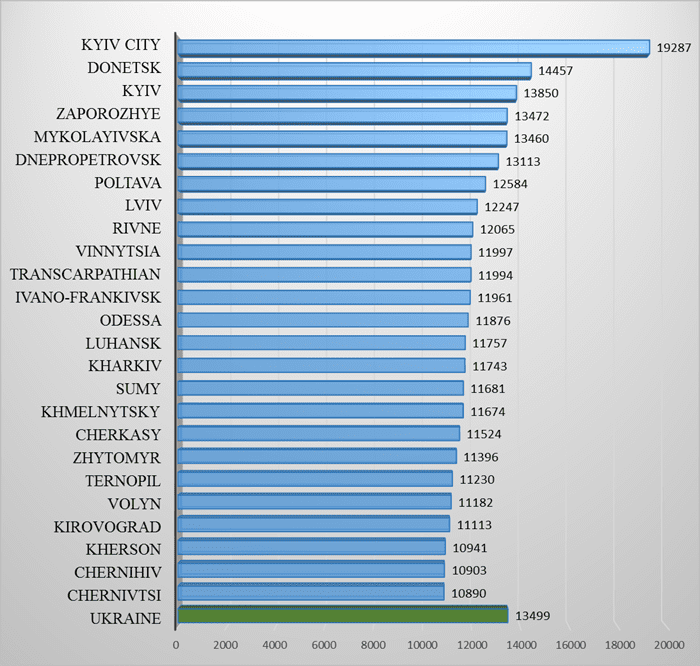
Average monthly wage by region in June 2021, UAH

Farmak pharmaceutical company, one of the largest exporters of pharmaceuticals, has opened an office in the United Arab Emirates, and plans to further strengthen its expansion in the Middle East and localization in Saudi Arabia and Egypt, its press service said on Tuesday. According to the press release, the company’s products have been presented in the Middle East market since 2016.
“While selling drugs through local distributors, the company audited the market potential and analyzed the product niches of the region’s pharmaceuticals. This year, in order to consolidate business management in the Middle East, Farmak’s management decided to open an office in the UAE,” the company said in the press release.
The new headquarters will be headed by Mourad I.Habib, who previously served as CEO of Tabuk Pharmaceuticals and led Sandoz’s Middle East operations for over eight years. According to Habib, who is quoted in the press release, the opening of the office in the UAE is only the first strategic step for Farmak; in the future, the company plans to strengthen its expansion in the region and localization in Saudi Arabia and Egypt.
The company said that at present, Farmak’s production facilities have successfully passed certification in the UAE government bodies. The company also registered a number of injectable drugs produced by Farmak, which are already being purchased at public and local hospital tenders in the region.
“The Middle East market is not new for us, and we are actively scaling up our presence in this region. We have chosen the United Arab Emirates as the center of activity in the Middle East,” Business Development Director of Farmak Viktor Kostiuk said.
At the same time, he recalled that the UAE has a reputation as a state with a very high level of the national healthcare system and strict regulation of activities related to the production and distribution of medicines. “The fact that Farmak products are represented on this market once again confirms the high level of quality of our products,” Kostiuk said.
Farmak is the leader of the Ukrainian pharmaceutical market. The company’s product portfolio includes more than 220 complex-component modern medicines. Among the main directions are endocrinological, gastroenterological, cardiological, neurological, anti-cold and other drugs.
The UAE office is the sixth international office of the company. In 2020, Farmak increased its export deliveries by 40%. Today, the manufacturer’s products are represented in more than 30 countries of the EU, Central and South America, the CIS, the Middle East and Asia.

Large international pharmaceutical companies are expecting the launch in Ukraine of a managed entry agreement mechanism (MEA), which will allow procuring innovative drugs using budget funds.
“We hope that MEA will start working this year,” Director of the health system development department at Roche Ukraine Maksym Proskurov told Interfax-Ukraine, recalling that the Cabinet of Ministers on January 27 of this year settled the procedure for conducting negotiations, concluding contracts and the MEA standard form.
Proskurov said that Roche is actively moving towards the conclusion of MEA. In particular, since April, the company has submitted four dossiers to the State Expert Center (SEC) for medical technology assessment, including three drugs for the treatment of cancer and one drug for the treatment of spinal muscular atrophy (SMA).
“An important basis for the MEA is passing the Health Technology Assessment (HTA) procedure, which actually scientifically substantiates the need to procure a drug based on the treatment effectiveness/price criterion. We expect that in September-October we will receive reports on the completion of the assessment,” he said.
At the same time, Proskurov drew attention to the fact that within the framework of the MEA “price discounts can be very different, but they will be significantly less than market discounts.” According to him, the key aspect when undergoing HTA is precisely the assessment of the impact on the budget, that is, the effective use of funds to provide as many patients as possible with innovative therapy within the rather limited Ukrainian budget.
“We hope that the first MEA will be concluded this year, since without this mechanism it is impossible to provide innovative medicines to patients with cancer and orphan diseases, such as SMA,” Proskurov said.
In turn, Head of the department for work with government bodies of Sanofi in Ukraine Natalia Baranovska said that in fact, the launch of the MEA mechanism is a long-awaited solution for many patients in Ukraine and the entire industry. “After all, MEA is a practice that has long been recognized all over the world and an effective tool for patients’ access to innovative drugs,” she said.
Baranovska said that Sanofi, as a world leader in the healthcare sector, has a wide practice of concluding such agreements with many countries in various therapeutic areas, including the treatment of orphan diseases.
At the same time, she said that it is for orphan diseases in Ukraine that “there are still several unresolved issues.” In particular, currently the legislation provides that for the feasibility of concluding a MEA, the HTA must be carried out. At the same time, the conduct of HTA of drugs intended for the treatment of orphan diseases “has many peculiarities.”
“Therefore, it is the issue of HTA for this group of diseases that has yet to be finalized by the Ministry of Health, and we, as a company, are ready to actively participate in its further discussion and refinement,” Baranovska said.
Minister of Health of Ukraine Viktor Liashko did not exclude that Ukraine is likely to introduce mandatory vaccination against coronavirus.
“I admit,” Liashko replied, answering the host’s question: “Do you admit compulsory vaccination?” on the air of the Freedom of Speech (Svoboda Slova) program on Monday.
The Minister said that currently in Ukraine vaccination is voluntary, but the government does not rule out some changes.
“Today, vaccination is free and voluntary. The question of the possibility of introducing certain categories of professions into the decreed ones is also being considered. This is a contingent that can come to work after a full course of vaccination against certain infectious diseases. Such options are being considered, but we have taken the experience of the EU and the U.S.: when restrictions are introduced, but they do not apply to vaccinated people,” Liashko said. In addition, the Minister allowed the possibility of vaccination among adolescents from 12 years of age.
“We expect and will launch a mass vaccination campaign among adolescents aged 12 and older. It’s time for everything,” he said.
According to him, priority will be given to people over 60, as 82% of all deaths are in this category.